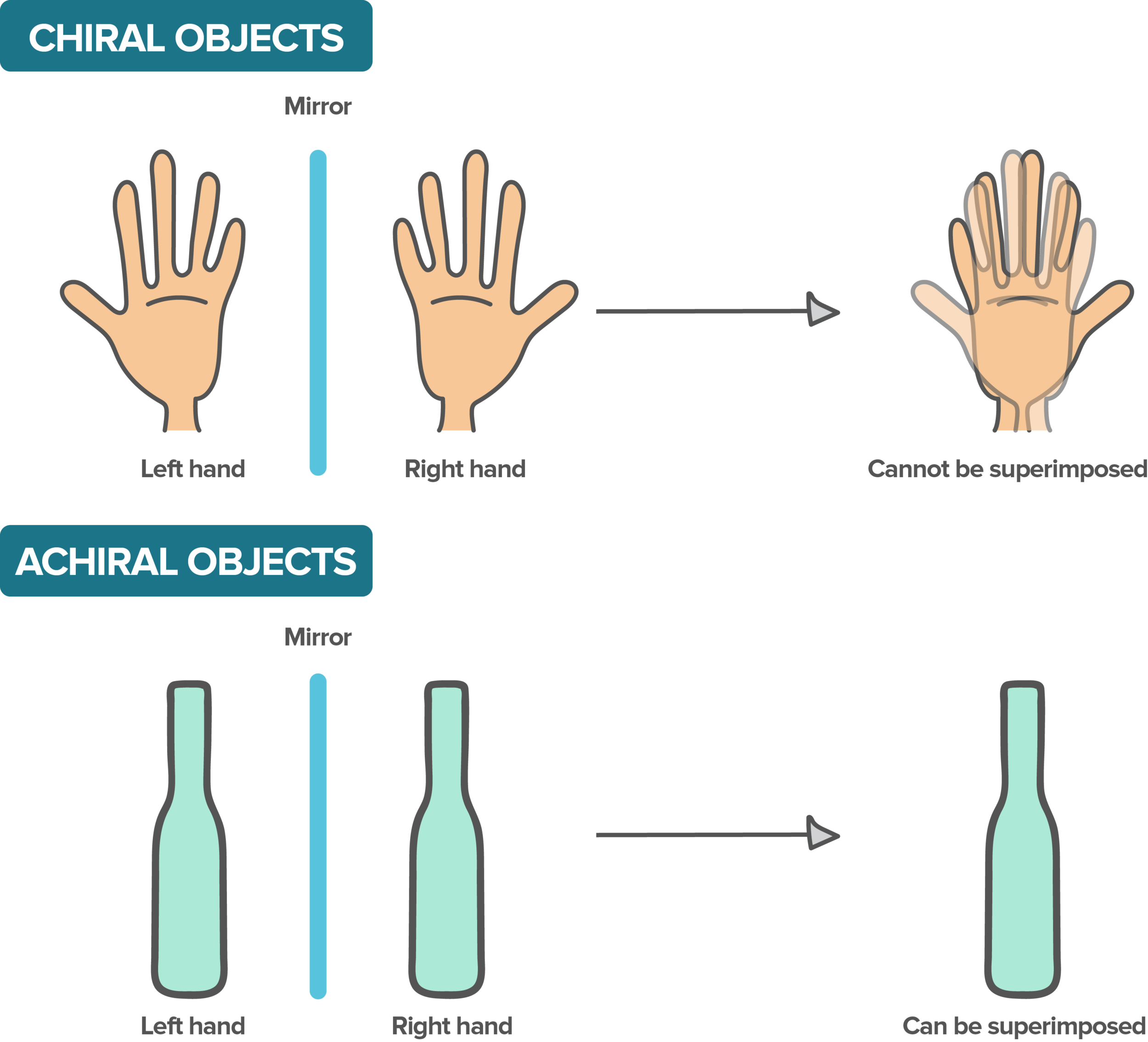
What is chirality?
Imagine putting a mirror between your two hands. Your left hand would be a mirror image to your right hand, and vice versa. However, try superimposing your hands, meaning you perfectly place your right hand on your left. It cannot be done. Either the thumbs will be on the wrong side, or the palms will be touching, which is wrong.
We can look at the same thing with molecules:
These can be superimposed, because if you rotate the left molecule 180 degrees, you get the right molecule. Now, we get to the definition of chiral.
Chiral
Its mirror image cannot be superimposed.
Achiral
its mirror image can be superimposed.
Stereocenters
- A stereocenter, or stereogenic center, is (in our case) a carbon atom bonded to four different constituents such that switching two would produce an enantiomer. I will get to what that means in a bit. First, a quick definition.A
What is a stereoisomer?
A stereoisomer is an isomer with the same molecular formula and sequence of bonded atoms, but the 3D shape is different. So, cis vs. trans is an example of a stereoisomer.
Rules for finding a stereocenter
- Must be bonded to four different things
- Cannot be internal (bonded to two hydrogens)
- Cannot be on the end
R vs. S
There are two ways of describing a stereocenter, R (right), clockwise, or S, counterclockwise.
How to determine R vs S
Assign the priority based on the atomic number. Look at the first atom bonded to the chiral center. If there is a tie, keep going until they differ. So if a Carbon is bonded to CH3, H, OH, and CH2CH3. Instantly, we know H is last (4), and OH is the greatest (1). Both other constituents have CH2, but the latter is connected to a carbon, so CH2CH3 is (2), and CH3 is (3).
Double Bonds
This is kind of tricky.
Pretty much, if it's a double bond, like C=C, we act like both atoms are bonded to two of the other. So, it'd be
C C
| |
C-C
(Sorry for the ugly image)
Last Rule of R vs. S
Finally, and this is extremely important, make sure the lowest priority is directed away from you.
Enantiomers and Diastereomers
Let's say the stereocenters of a molecule are R,S,S. The enantiomer is the polar opposite. It's S,R,R. You can switch from R to S, or vice versa, by switching whether the bond goes into or out of the page. A diastereomer is anything that isn't R,S,S, but isn't the polar opposite. So, S,S,S, would be a diastereomer.
Cis/Trans Bonds
This just describes whether the hydrogens are on the same side. If they are, it's cis. If not, it's trans.
E/Z Bonds
Numbers ordered by atomic number.
This is a generalization of cis/trans bonds.
Here, you order the constituents by atomic number, just like we did for R vs S. In addition, you keep going until you cannot break a tie. If the higher priority constituents are on the same side, as is the case with the molecule above, it is (Z)ame. (Z = Zusammen, German for "together"). If they're on the other side, it's E (E = entgegen, German for "opposite").